February, 2020
now browsing by month
Interim Clinical Guidance for Management of Patients with Confirmed 2019 Novel Coronavirus (Covid-19) Infection-www.cdc.gov
https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
Summary of Recent Changes
Revisions were made on February 12, 2020, to reflect the following:
- Information added regarding time of illness onset to hospital admission
- Information added on the detection of 2019-nCoV in extrapulmonary specimens
- Clarification of the type of advanced support observed among hospitalized patients
- Interim guidance for discontinuation of transmission-based precautions and in-home isolation
Updated February 12, 2020
This interim guidance is for clinicians caring for patients with confirmed 2019 novel coronavirus (2019-nCoV) infection. This update includes additional information regarding time from illness onset to hospital admission, detection of 2019-nCoV in extrapulmonary specimens, clarifies the type of advanced support observed among hospitalized patients and provides interim guidance for discontinuation of transmission-based precautions and in-home isolation. CDC will update this interim guidance as more information becomes available.
Clinical Presentation
There are a limited number of reports that describe the clinical presentation of patients with confirmed 2019-nCoV infection, and most are limited to hospitalized patients with pneumonia. The incubation period is estimated at ~5 days (95% confidence interval, 4 to 7 days). [1] Some studies have estimated a wider range for the incubation period; data for human infection with other coronaviruses (e.g. MERS-CoV, SARS-CoV) suggest that the incubation period may range from 2-14 days. Frequently reported signs and symptoms include fever (83–98%), cough (46%–82%), myalgia or fatigue (11–44%), and shortness of breath (31%) at illness onset. [2–4] Sore throat has also been reported in some patients early in the clinical course. Less commonly reported symptoms include sputum production, headache, hemoptysis, and diarrhea. Some patients have experienced gastrointestinal symptoms such as diarrhea and nausea prior to developing fever and lower respiratory tract signs and symptoms. The fever course among patients with 2019-nCoV infection is not fully understood; it may be prolonged and intermittent. Asymptomatic infection has been described in one child with confirmed 2019-nCoV infection and chest computed tomography (CT) abnormalities. [5]
Risk factors for severe illness are not yet clear, although older patients and those with chronic medical conditions may be at higher risk for severe illness. Most reported cases have occurred in adults (median age 59 years).[1] In one study of 425 patients with pneumonia and confirmed 2019-nCoV infection, 57% were male. [1] Approximately one-third to one-half of reported patients had underlying medical comorbidities, including diabetes, hypertension, and cardiovascular disease. [2–3] In another study, compared to patients not admitted to an intensive care unit, critically ill patients were older (median age 66 years versus 51 years), and were more likely to have underlying co-morbid conditions (72% versus 37%). [3]
Clinical Course
Clinical presentation among reported cases of 2019-nCoV infection varies in severity from asymptomatic infection or mild illness to severe or fatal illness. Some reports suggest the potential for clinical deterioration during the second week of illness.[2] In one report, among patients with confirmed 2019-nCoV infection and pneumonia, just over half of patients developed dyspnea a median of 8 days after illness onset (range: 5–13 days). [2] In another report, the mean time from illness onset to hospital admission with pneumonia was 9 days.[1]
Acute respiratory distress syndrome (ARDS) developed in 17–29% of hospitalized patients, and secondary infection developed in 10%. [2,4] In one report, the median time from symptom onset to ARDS was 8 days.[3] Between 23–32% of hospitalized patients with 2019-nCoV infection and pneumonia have required intensive care for respiratory support.[2–3] In one study, among critically ill patients admitted to an intensive care unit, 11% received high-flow oxygen therapy, 42% received noninvasive ventilation, and 47% received mechanical ventilation. [3] Some hospitalized patients have required advanced organ support with endotracheal intubation and mechanical ventilation (4–10%), and a small proportion have also been supported with extracorporeal membrane oxygenation (ECMO, 3–5%).[3–4] Other reported complications include acute cardiac injury, arrhythmia, shock, and acute kidney injury. Among hospitalized patients with pneumonia, the case fatality proportion has been reported as 4–15%.[2–4] However, as this estimate includes only hospitalized patients it is biased upward. Nosocomial transmission among healthcare personnel and patients has been reported.
Diagnostic Testing
Information on specimen collection, handling, and storage is available at: Real-Time RT-PCR Panel for Detection 2019-Novel Coronavirus. After initial confirmation of 2019-nCoV infection, additional testing of clinical specimens can help inform clinical management, including discharge planning.
Laboratory and Radiographic Findings
The most common laboratory abnormalities reported among hospitalized patients with pneumonia on admission included leukopenia (9–25%), leukocytosis (24–30%), lymphopenia (63%), and elevated alanine aminotransferase and aspartate aminotransferase levels (37%). [2,4] Most patients had normal serum levels of procalcitonin on admission. Chest CT images have shown bilateral involvement in most patients. Multiple areas of consolidation and ground glass opacities are typical findings reported to date. [2–4, 6–9]
Limited data are available about the detection of 2019-nCoV and infectious virus in clinical specimens. 2019-nCoV RNA has been detected from upper and lower respiratory tract specimens, and the virus has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid. 2019-nCoV RNA has been detected in blood and stool specimens, but whether infectious virus is present in extrapulmonary specimens is currently unknown. The duration of 2019-nCoV RNA detection in the upper and lower respiratory tracts and in extrapulmonary specimens is not yet known. It is possible that RNA could detected for weeks, which has occurred in some cases of MERS-CoV or SARS-CoV infection.[9–18] Viable SARS-CoV has been isolated from respiratory, blood, urine, and stool specimens. In contrast, viable MERS-CoV has only been isolated from respiratory tract specimens. [18–20]
Clinical Management and Treatment
Healthcare personnel should care for patients in an Airborne Infection Isolation Room (AIIR). Standard Precautions, Contact Precautions, and Airborne Precautions with eye protection should be used when caring for the patient. See Interim Health Care Infection Prevention and Control Recommendations for Patients Under Investigation for 2019 Novel Coronavirus.
Patients with a mild clinical presentation may not initially require hospitalization. However, clinical signs and symptoms may worsen with progression to lower respiratory tract disease in the second week of illness; all patients should be monitored closely. Possible risk factors for progressing to severe illness may include, but are not limited to, older age, and underlying chronic medical conditions such as lung disease, cancer, heart failure, cerebrovascular disease, renal disease, liver disease, diabetes, immunocompromising conditions, and pregnancy.
The decision to monitor a patient in the inpatient or outpatient setting should be made on a case-by-case basis. This decision will depend not only on the clinical presentation, but also on the patient’s ability to engage in monitoring, home isolation, and the risk of transmission in the patient’s home environment. For more information, see Criteria to Guide Evaluation of Patients Under Investigation (PUI) for 2019-nCoV.
No specific treatment for 2019-nCoV infection is currently available. Clinical management includes prompt implementation of recommended infection prevention and control measures and supportive management of complications, including advanced organ support if indicated.
Corticosteroids should be avoided unless indicated for other reasons (for example, chronic obstructive pulmonary disease exacerbation or septic shock per Surviving Sepsis guidelinesexternal icon), because of the potential for prolonging viral replication as observed in MERS-CoV patients. [12, 21–23]
For more information, see: WHO interim guidance on clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspectedpdf iconexternal icon and Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of Americaexternal icon.
Investigational Therapeutics
There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration (FDA) to treat patients with 2019-nCoV infection. Some in-vitro or in-vivo studies suggest potential therapeutic activity of compounds against related coronaviruses, but there are no available data from observational studies or randomized controlled trials in humans to support recommending any investigational therapeutics for patients with confirmed or suspected 2019-nCoV infection at this time. Remdesivir, an investigational antiviral drug, was reported to have in-vitro activity against 2019-nCoV. [24] A small number of patients with 2019-nCoV infection have received intravenous remdesivir for compassionate use outside of a clinical trial setting. A randomized placebo-controlled clinical trial of remdesivir for treatment of hospitalized patients with pneumonia and 2019-nCoV infection has been implemented in China. A randomized open label trial of combination lopinavir-ritonavir treatment has been also been conducted in hospitalized patients with pneumonia and 2019-nCoV infection in China, but no results are available to date. Clinical trials of other potential therapeutics for 2019-nCoV infection are being planned. For information on specific clinical trials underway for treatment of patients with 2019-nCoV infection, see clinicaltrials.govexternal icon.
Interim Guidance for Discontinuing Transmission-based Precautions or In-Home Isolation for Persons with Laboratory-confirmed 2019-nCoV Infection*
Standard and Transmission-based precautions (i.e., Contact and Airborne precautions with eye protection) should be used for persons with laboratory-confirmed 2019-nCoV infection. This guidance applies to patients being managed in a hospital in an airborne infection isolation room (AIIR) and to patients being cared for in-home isolation.
Decisions to discontinue transmission-based precautions or in-home isolation can be made on a case-by-case basis in consultation with clinicians, infection prevention and control specialists, and public health based upon multiple factors, including disease severity, illness signs and symptoms, and results of laboratory testing for 2019-nCoV in respiratory specimens.
See: Interim Considerations for Disposition of Hospitalized Patients with 2019-nCoV Infection
Additional resources:
- Interim Guidance for Healthcare Professionals
- Resources for Hospitals and Healthcare Professionals Preparing for Patients with Suspected or Confirmed 2019-nCoV
- Interim Health Care Infection Prevention and Control Recommendations for Persons Under Investigation for 2019 Novel Coronavirus
- World Health Organization. Interim Guidance on Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspectedexternal icon
- American Thoracic Society and Infectious Diseases Society of America Clinical Practice Guidelines. Diagnosis and treatment of adults with community-acquired pneumoniaexternal icon
- Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016external icon
- Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaexternal icon
References Click
Page last reviewed: February 12, 2020 Content source: National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases
IMAGES: What New Coronavirus Looks Like Under The Microscope by npr.org
https://www.livescience.com/new-coronavirus-images.html
IMAGES: What New Coronavirus Looks Like Under The Microscope
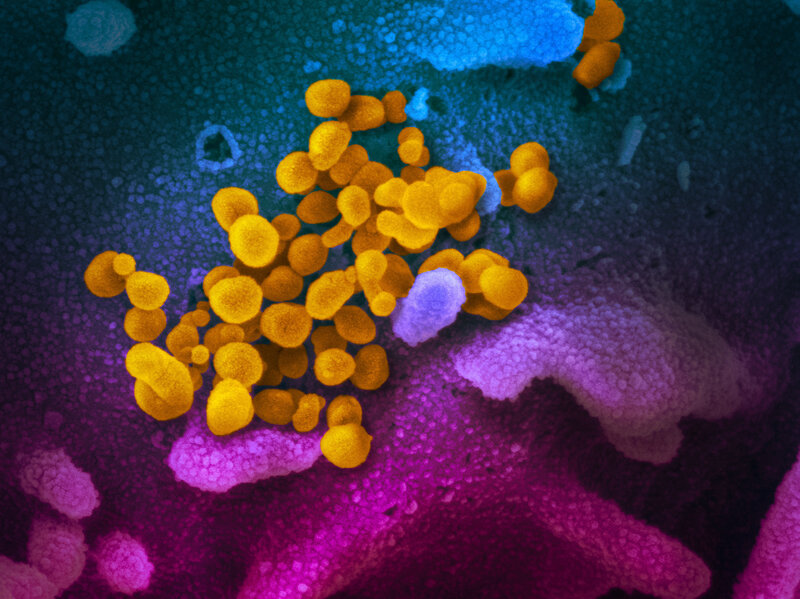
COVID-19 coronavirus is seen in yellow, emerging from cells (in blue and pink) cultured in the lab. This image is from a scanning electron microscope.NIAID-RML
The images of the current outbreak of the new coronavirus have so far been very human: air travelers wearing masks, tourists stranded on cruise ships, medical workers wearing protective suits.
But new images of the virus show us what it looks like up close.
These images were made using scanning and transmission electron microscopes at the National Institute of Allergy and Infectious Diseases’ Rocky Mountain Laboratories in Hamilton, Mont. NIAID is part of the National Institutes of Health.
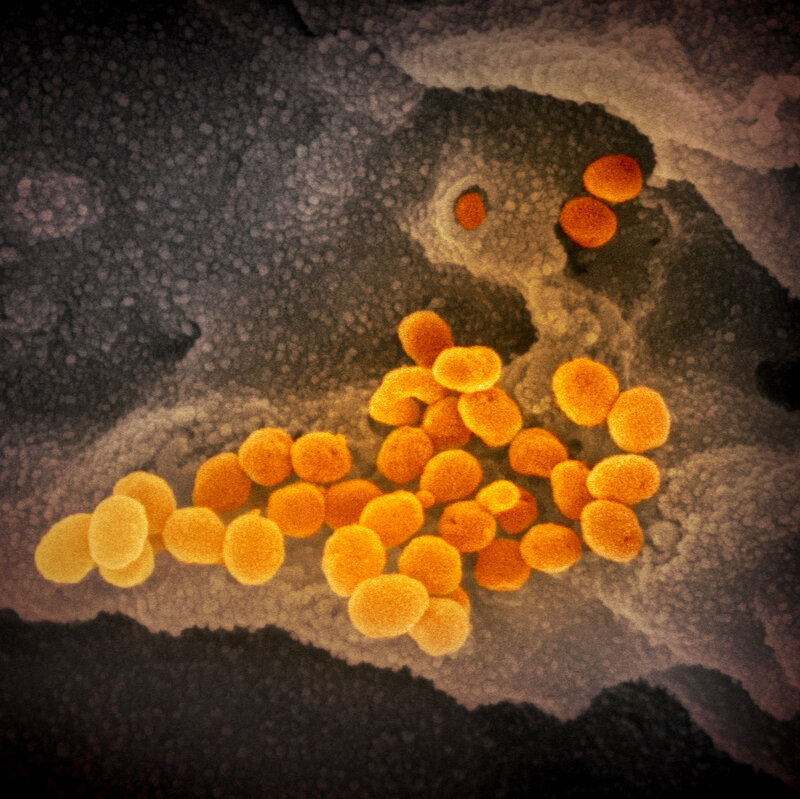
This image from a scanning electron microscope shows, in orange, the coronavirus that causes the disease COVID-19. The virus was isolated from a patient in the U.S. and is seen here emerging from the surface of cells — in gray — cultured in the lab.NIAID-RML
Emmie de Wit, chief of NIAID’s Molecular Pathogenesis Unit, provided the virus samples. Microscopist Elizabeth Fischer produced the images, and the lab’s visual medical arts office digitally colorized the images.

In this image from a scanning electron microscope, the new coronavirus is in orange.NIAID-RML
NIAID notes that the images look rather similar to previous coronavirus MERS-CoV (Middle East respiratory syndrome coronavirus, which emerged in 2012) and the original SARS-CoV (severe acute respiratory syndrome coronavirus, which emerged in 2002).
“That is not surprising: The spikes on the surface of coronaviruses give this virus family its name – corona, which is Latin for ‘crown,’ and most any coronavirus will have a crown-like appearance,” the institute explains in a blog post.
The Nuffield Council on Bioethics’ report Research in global health emergencies: ethical issues (published January 2020).
https://www.nuffieldbioethics.org/assets/pdfs/RGHE_Short_report_web_version1.pdf
Contents
2-3 Why is this report needed?
4-5 Emergency preparedness, response, and research
6-7 Developing an ethical compass
8 Whose voices should be heard? – An inclusive approach to influencing research
9 An inclusive approach to study design and review
10 Consent and beyond – the wider ethics ecosystem
11 Collaborations and partnerships
12 Data and samples
13 Ethical issues faced by front-line workers
Infection prevention and control during health care when novel coronavirus (COVID-19) infection is suspected
Overview
This is the first edition of guidance on infection prevention and control (IPC) strategies for use when infection with a novel coronavirus (2019-nCoV) is suspected. It has been adapted from WHO’s Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection,1 based on current knowledge of the situation in China and other countries where cases were identified and experiences with severe acute respiratory syndrome (SARS)-CoV and MERS-CoV. 2
WHO will update these recommendations as new information becomes available.
This guidance is intended for healthcare workers (HCWs), healthcare managers and IPC teams at the facility level but it is also relevant for the national and district/provincial level. Full guidelines are available from WHO.2
Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: interim guidance, updated October 2019. Geneva: World Health Organization; 2019 (WHO/MERS/IPC/15.1 Rev. 1; https://apps.who.int/iris/handle/10665/174652, accessed 17 January 2020).
Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care: WHO guidelines. Geneva: World Health Organization; 2014 (http://apps.who.int/iris/10665/112656, accessed 17 January 2020).
Novel Coronavirus(2019-nCoV) Situation Reports
https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
Globally 11953 confirmed (2128 new)
China
11821 confirmed (2102 new)
1795 severe (268 new)
259 deaths (46 new)
Outside of China
132 confirmed (26 new) 23 countries (4 new)




